Day 1
1. Introduction to molecular biology
Molecular biology is a discipline at the intersection between biochemistry and cellular biology:
- Molecular biology, per formal definition, studies nucleic acids but in general deals with the interactions among bio-macromolecules
- Biochemistry, per definition, studies proteins, but in general deals with the metabolic interactions in a cell, often involving macro and micro-molecules
- Cellular biology studies the organization and macro-processes that occur within a cell
Molecular biology was founded with the discovery of the DNA double helix structure (1953, Watson and Crick), and escalated quickly with several techniques introduced during the 70s, the 80s and the 90s, such as recombinant DNA, genetic engineering and all the procedures that allow to isolate and manipulate the genetic material.
Several disciplines, like bioinformatics, structural biology, molecular genetics, molecular evolution, transcriptomics and proteomics are theoretically and practically supported by molecular biology.
2. Nucleobases, nucleosides and nucleotides
2a. Nucleobases
Nucleobases are organic molecules based on heterocyclic structures, meaning that they have a close structure in which are included also heteroatoms, such as oxygen (O), nitrogen (N) or sulfur (S). In the case of nucleobases, we have N as heteroatom:
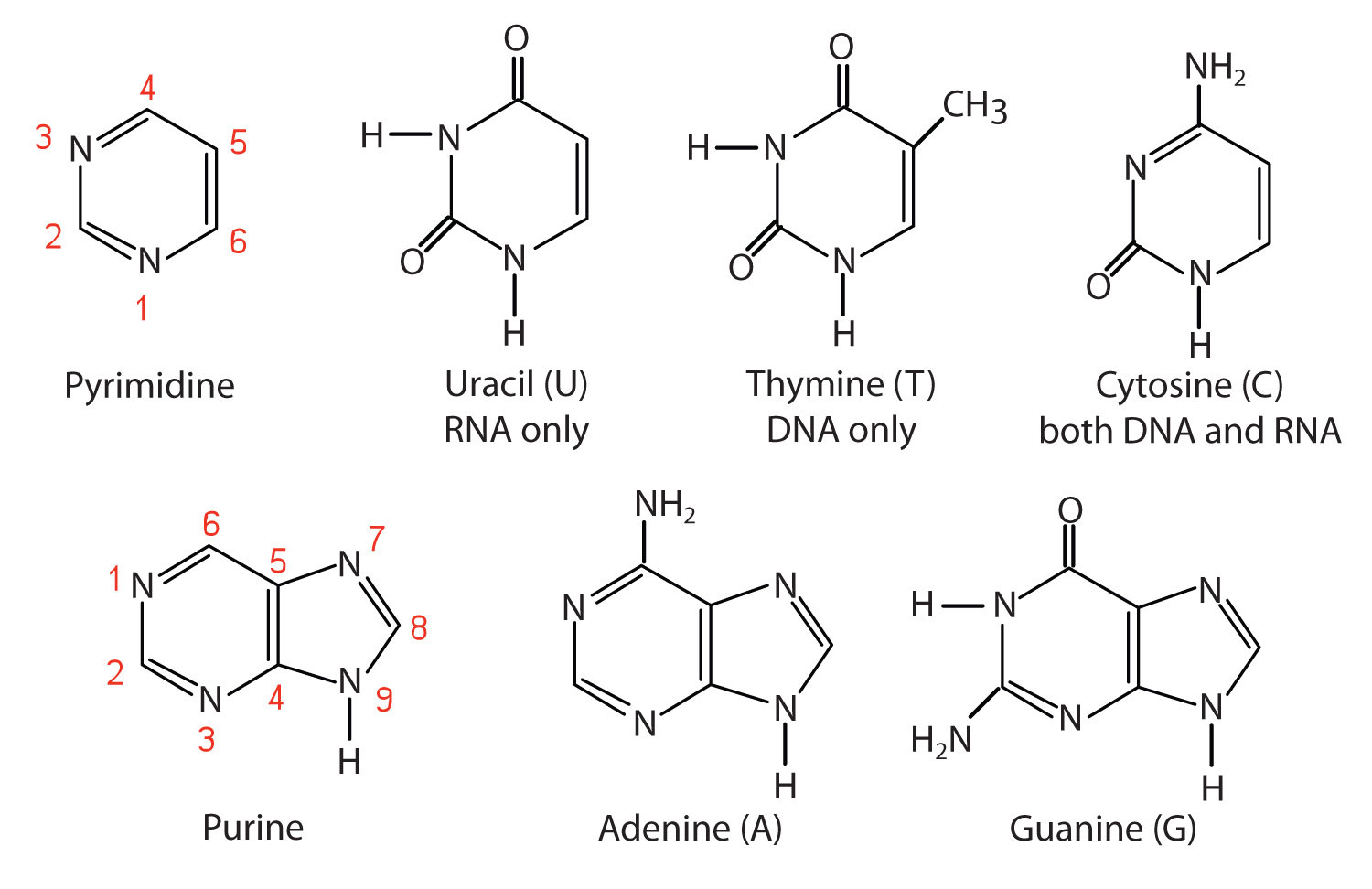
As you can see from the image, we have 5 "traditional" nucleobases, divided in two classes:
- Pyrimidines: one hexagonal rings, with two Ns, in position 1 and 3. Belonging to this class we have uracil, thymine and cytosine
- Purines: one hexagonal ring concatenated to a pentagonal one, with 4 Ns (1,3,7,9). Belonging to this class we have adenine and guanine As you can see, there is an interesting feature in the N-1 and N-9 of purines and pyrimidines: they are bound to a H. Let's keep an eye on this!
2b. Nucleosides
Nucleosides are nucleobases linked to a pentose sugar. The pentose sugar can be:

We notice two things from this image:
- Ribose has a hydroxyl group in position 2', whereas deoxyribose does not (hence the deoxy-)
- The numbering of the carbons has a
'(prime): this is due to the fact that these carbons are also linked to another carbon-based molecule (a nucleobase), and we need to differentiate them
Ribose is the pentose employed for RNA synthesis, while deoxyribose for DNA: the huge difference in the stability of the two molecules (DNA hyper-stable, RNA easily degraded) boils down essentially to the greater stability of the pentose without a hydroxyl in 2'.
Nucleosides link a nucleobase to a pentose via N-glycosdic bond, which occurs between the 2' carbon of the pentose and the N-1 (pyrimidine) or N-9 (purine) of the nucleobase. Bonding happens through a condensation reaction
2c. Nucleotides
Nucleotides are, formally, n-phospho-nucleosides, meaning that they "only" add (max. 3) phosphate groups to the 5' end of the nucleoside. The 5' end indeed is easily subject to a condensation reaction involving its hydroxyl group and the same group in HPO4- ion.
This reaction generates a mono-phospho-nucleoside, that can be further condensed into a di-phosoho-nucleoside and a tri-phospho-nucleoside, which are progressively more subject to repulsion forces among negative charges in the phosphate groups and thus more unstable (hence more energy dense, that's why ATP is an energy-related molecule).
Nucleotides in the nucleic acids are mono-phospho-nucleotides, but they themselves come from tri-phospho-nucleotides synthetized by the metabolic pathway of nucleotide biosynthesis. In order to form RNA, we need a tri-phospho-nucleoside (NTP), while for DNA we need a tri-phosphate-deoxynucleoside (dNTP).
3. Nomenclature
| Nucleobase | Nucleoside | Nucleotide | RNA | DNA |
|---|---|---|---|---|
| Adenine (A) | (deoxy-)Adenosine | (5'-deoxy-)Adenosine-n-phosphate (AMP/ADP/ATP) or Adenylic acid | ✅ | ✅ |
| Guanine (G) | (deoxy-)Guanosine | (5'-deoxy-)Guanosine-n-phosphate (GMP/GDP/GTP) or Guanylic acid | ✅ | ✅ |
| Thymine (T) | (deoxy-)Thymidin | (5'-deoxy-)Thymidin-n-phosphate (TMP/TDP/TTP) or Thymidylic acid | ❌ | ✅ |
| Cytosine (C) | (deoxy-)Cytidin | (5'-deoxy-)Cytidin-n-phosphate (CMP/CDP/CTP) or Cytidylic acid | ✅ | ✅ |
| Uracil (U) | (deoxy-)Uridin | (5'-deoxy-)Uridin-n-phosphate (AMP/ADP/ATP) or Uraciyic acid | ✅ | ❌ |